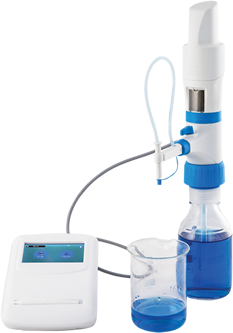
The use of high-quality precision liquid handling instruments throughout the drug discovery, testing, and production processes gives scientists and laboratory technicians a sense of confidence in their procedures. However, this high-quality instrument needs regular calibration technologies to ensure precise measurement and transfer volumes of liquid to obtain credible results.
Even minute discrepancies in the amount of acid, solvent or any other reagent dispensed may affect the outcome and reproducibility of the experiments. Therefore, it is vital to check the calibration of the pipette at least every six months. There are various options available to test the calibration of pipettes and measure their efficacy. In this post, we will compare Gravimetric testing and Photometric testing for pipettes.
Gravimetric Testing
Gravimetric testing is the most common and economical method used for pipette calibration. In this process, an aliquot of distilled water is placed in a vessel, and its weight is measured using an analytical balance. The weight is then converted to mass and then to volume using standard conversion factors or produced by software packages. For example, one of the standard factors is the Z factor, which enables the conversion of the weight of the liquid on the analytical balance into volume by taking into consideration the humidity, temperature, and other environmental factors.
Gravimetry is used due to its simplicity, accuracy, and traceability. Moreover, it is a well-accepted technology and is recognized by national and international regulatory agencies such as the International Organization for Standardization (ISO), the College of American Pathologists (CAP), and ASTM International. The standards of gravimetry testing are ISO 8655-6 and ASTM E1154.
Gravimetry is a method of choice to calibrate the pipettes but has a few drawbacks. It is affected by various environmental conditions like evaporation, temperature, static electricity, and vibration. The resulting density of the liquid in these situations is significantly different from that of the testing environment, and data may not conform to expected results. Furthermore, as water is used for gravimetric testing, it does not ensure proper performance when using other liquids with different capillary pressures or surface tensions. Thus, the calibration is usually performed in an environment similar to the one where the pipette will ultimately be used.
Photometric Testing
The photometric calibration of pipettes has grown and improved significantly in recent years. ISO 8655-7 has approved the use of the photometric testing method for pipettes performance verification. This method uses light absorption to verify volume accuracy with a photometer and is used for low volume measurements and high throughput screening applications. In this method, a stable dye solution is delivered with a pipette into a microtitre plate. A specified wavelength beam of light is passed through the solution and the photometer measures the quantity of light that passes through. The amount of light absorbed is proportional to the amount of dye present.
Photometric testing can be implemented using two different procedures – Single-Dye and Ratiometric. Single-dye photometric testing provides good precision measurement and is less sensitive to environmental conditions. Like all dye-based methods, photometric methods must be correctly standardized to get quantitative results for accurate measurements.
The Ratiometric photometry is a refinement to single-dye photometry as it overcomes the accuracy limitations. This method uses two standardized dyes, and the measurement process produces absorbance readings in pairs which are combined into absorbance ratio readings. The primary advantage of this method is its ability to improve the accuracy and robustness of measurement. Absorbance ratios are measured more accurately than individual absorbances. Thus, leading to a higher degree of accuracy and precision in the ratiometric method.
Compared to gravimetry testing, photometric testing offers a great speed, ease of use, and enhanced accuracy.
Conclusion
Pharmaceutical laboratories have varying protocols, processes, and requirements that affect the choice of calibration technologies for liquid handling devices. Gravimetry testing and photometry testing are common testing methods to calibrate pipettes. Understanding the assay and laboratory quality requirements, traceability needs, tolerance for error, and level of pipette precision and accuracy calculation can help laboratories make the right decision.





 16937
16937