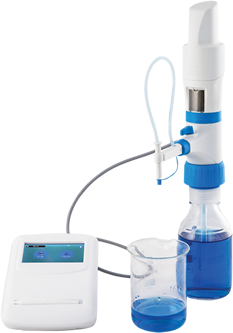
In the realm of quality management and calibration standards, ISO norms play a pivotal role in ensuring precision, accuracy, and reliability across various industries. Among these, ISO 8655 and ISO 17025 stand out as critical benchmarks, each serving distinct yet interconnected purposes. In this comprehensive guide, we delve into the disparities between ISO 8655 and ISO 17025, shedding light on their unique features, applications, and significance.
ISO 8655: Precision in Liquid Handling
ISO 8655 specifically pertains to the calibration and use of piston-operated volumetric apparatus, commonly employed in laboratories for precise liquid handling. This standard meticulously outlines requirements for the design, calibration, and operation of these instruments, aiming to guarantee accuracy and consistency in volumetric measurements.
Key Components of ISO 8655
- Instrument Design: ISO 8655 sets forth stringent criteria for the construction and design of piston-operated pipettes, burettes, and dispensers. It delineates specifications regarding materials, dimensions, and ergonomic considerations to optimize functionality and user comfort.
- Calibration Protocols: Central to ISO 8655 is the establishment of robust calibration procedures tailored to ensure traceability and repeatability in volume measurements. Calibration methods encompass gravimetric, photometric, and spectrophotometric techniques, with emphasis on meticulous documentation and validation.
- Quality Assurance: Adherence to ISO 8655 necessitates comprehensive quality assurance measures throughout the lifecycle of volumetric apparatus. This encompasses rigorous testing, maintenance, and performance verification to mitigate uncertainties and uphold measurement integrity.
- Training and Competence: Recognizing the critical role of operators in maintaining measurement accuracy, ISO 8655 underscores the importance of personnel training and competence assessment. Competent personnel are essential for the proficient operation, calibration, and maintenance of volumetric instruments.
ISO 17025: Accreditation for Testing and Calibration Laboratories
In contrast to the instrument-specific focus of ISO 8655, ISO 17025 addresses broader quality management principles applicable to testing and calibration laboratories. This standard serves as a globally recognized framework for demonstrating technical competence and ensuring the reliability of laboratory results.
Key Components of ISO 17025
- Scope of Accreditation: ISO 17025 necessitates a comprehensive scope of accreditation, delineating the types of tests, calibrations, and measurements covered by the laboratory’s quality management system. This ensures transparency and clarity regarding the laboratory’s capabilities and limitations.
- Technical Competence: Central to ISO 17025 is the demonstration of technical competence through proficiency testing, inter-laboratory comparisons, and participation in relevant proficiency schemes. Laboratories must exhibit proficiency in specific testing or calibration methods within their accredited scope.
- Quality Management System (QMS): ISO 17025 mandates the implementation of a robust quality management system tailored to the laboratory’s operations. This encompasses document control, internal audits, corrective actions, and continual improvement initiatives to enhance overall efficiency and reliability.
Traceability and Measurement Uncertainty: Ensuring traceability to international standards and minimizing measurement uncertainty are fundamental tenets of ISO 17025. Laboratories must establish and maintain traceable measurement systems while quantifying and controlling sources of uncertainty to uphold result reliability.
Distinguishing Between ISO 8655 and ISO 17025
While both ISO 8655 and ISO 17025 contribute to quality assurance within the laboratory environment, they serve distinct purposes and address different aspects of measurement integrity. Differentiating factors include:
- Scope: ISO 8655 pertains specifically to the calibration and use of volumetric apparatus, whereas ISO 17025 encompasses broader quality management principles applicable to testing and calibration laboratories.
- Focus: ISO 8655 focuses on the design, calibration, and operation of piston-operated volumetric instruments, emphasizing precision and accuracy in liquid handling. In contrast, ISO 17025 emphasizes technical competence, quality management systems, and result reliability across various laboratory activities.
- Applicability: ISO 8655 is directly applicable to laboratories utilizing piston-operated pipettes, burettes, and dispensers for liquid handling tasks. Conversely, ISO 17025 applies to all testing and calibration laboratories seeking accreditation and recognition of their technical competence.
- Interconnection: While distinct, Both standards are interrelated in practice, as adherence to ISO 8655 may contribute to compliance with ISO 17025 requirements within the scope of calibration activities.
In summary, both represent indispensable standards in ensuring measurement accuracy, reliability, and quality within laboratory settings. While ISO 8655 focuses on precision in liquid handling through stringent calibration protocols, ISO 17025 provides a comprehensive framework for demonstrating technical competence and maintaining quality management systems across diverse laboratory operations. Understanding the distinctions between these standards is essential for laboratories seeking to uphold the highest standards of measurement integrity and reliability.
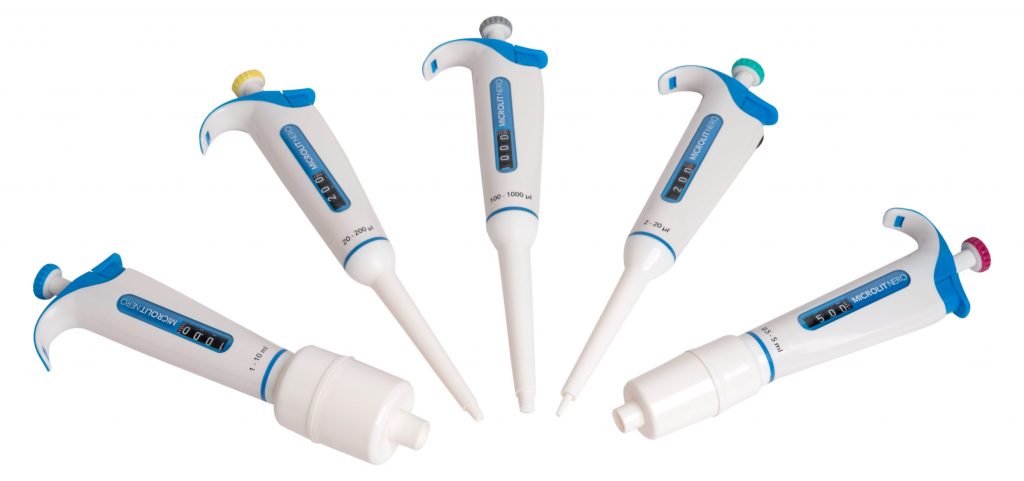
Microlit’s portfolio of laboratory instruments and consumables, including the award-winning Bottletop Dispensers, Micropipettes, E-Burettes, Pipette Controller, etc., exemplifies excellence in precision and quality. Each product is meticulously engineered to adhere to both standards, ensuring unparalleled accuracy and reliability in your lab experiments.
With Microlit, you can trust in the precision of your measurements, backed by our unwavering commitment to meeting international quality benchmarks. Elevate your laboratory performance with Microlit’s range of products, where precision meets perfection, setting a new standard in laboratory instrumentation.
To know more about the Microlit product range, visit the website www.microlit.us.





 2254
2254