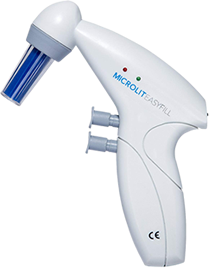
Titration is a method of chemical qualitative analysis used to determine an analyte’s concentration in a mixture. Another name for titration, volumetric analysis, is a crucial method in analytical chemistry. It involves preparing a titrant, which is a standard solution with a known volume and concentration. This titrant is gradually added to the analyte, or solution under analysis, until the reaction reaches an endpoint or equivalence point. At this point, the amount of titrant consumed can be used to calculate the analyte’s concentration.
There are many types of titrations with different goals and procedures. However, the most common types of it in quantitative chemical analysis are redox titrations and acid-base titrations.
These are the four common titrations:
- Acid-base titrations: Acid-base titration mainly depends on the neutralization between acid and base when mixed in solution. More significantly, the strength of an acid is determined using a standard solution of a base. This process is also called acidimetry.
- Redox titrations: These are almost similar to the volumetric acid. Base titrations except that here, the reactions involved are Redox reactions.
- Precipitation titrations: Precipitation titration is based on insoluble precipitation where two reacting substances are brought in contact, which is called precipitation titration. The titrant reacts with the analyte and forms an insoluble material. It continues until the analysis is completely over. When the titrant is high it reacts with the indicator and signals to end the titration process.
- Complexometric titrations: The complexometric titration is where an undivided complex is formed at an equivalence point. This is greater than the precipitation titrations, and there will be no error due to co-precipitation.




 13836
13836