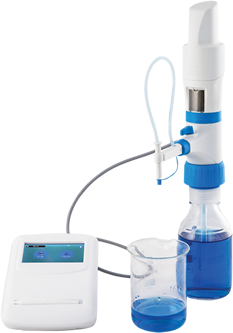
Vaccine development represents a pivotal aspect of public health, requiring rigorous methodologies to ensure the safety and efficacy of biologics. Two critical components that underpin the success of this process are environmental monitoring and liquid handling. As the complexity of vaccine formulation increases and the demand for rapid production escalates, it becomes essential to employ advanced technical strategies that streamline these functions. This blog explores the intricate interplay between environmental monitoring systems and liquid handling technologies, highlighting their collective role in optimizing vaccine development.
The Critical Role of Environmental Monitoring in Vaccine Development
Environmental monitoring is an essential element in vaccine manufacturing, aimed at mitigating risks associated with contamination and ensuring compliance with regulatory standards such as Good Manufacturing Practices (GMP). The primary objective is to maintain aseptic conditions within controlled environments, thus preserving the integrity of the vaccine product.
Key Parameters for Environmental Monitoring
- Microbial Contamination Assessment:Implementing a robust microbial monitoring program involves the use of environmental samplers, such as settle plates, active air samplers, and contact plates, to quantitatively assess bioburden levels. Real-time PCR (Polymerase Chain Reaction) and culture-based methods can be utilized for rapid detection of pathogens, facilitating immediate corrective actions when contamination is detected.
- Particulate Matter Control:The presence of particulates can lead to adverse reactions in vaccine formulations. Utilizing laser diffraction particle size analyzers and optical particle counters enables the assessment of airborne particulate levels. Continuous monitoring systems can trigger alerts if predefined thresholds are exceeded, prompting immediate investigations.
- Temperature and Humidity Regulation:Vaccines are often sensitive to temperature fluctuations, requiring stringent monitoring of both ambient and surface temperatures. The use of calibrated data loggers and wireless monitoring systems allows for real-time tracking of these parameters, ensuring compliance with storage requirements throughout the production cycle.
- Pressure Differentials:Cleanrooms are designed to operate under positive pressure to prevent ingress of contaminants. Continuous monitoring of differential pressure using pressure sensors helps maintain the integrity of the controlled environment. The implementation of alarm systems that alert personnel to pressure deviations is crucial for immediate rectification.
The Integration of Automation in Environmental Monitoring
Manual environmental monitoring methods are increasingly being supplanted by automated systems that provide continuous, real-time data acquisition. Automation not only minimizes human error but also enhances data integrity and traceability. Advanced environmental monitoring systems can be integrated with Building Management Systems (BMS) to allow for centralized control and monitoring, ensuring that all parameters remain within specified limits.
Precision in Liquid Handling Processes
Liquid handling is a foundational component of vaccine production, encompassing the accurate dispensing and transfer of biological fluids, reagents, and culture media. The precision of liquid handling directly influences the reproducibility and reliability of vaccine formulations.
Liquid Handling Techniques in Vaccine Development
- Automated Pipetting Systems:High-throughput automated pipetting systems, equipped with advanced software for method development and data tracking, allow for the precise dispensing of reagents at micro- and nanoliter scales. Such systems are critical in assays where minute volume differences can impact biological activity.
- Microplate-Based Assays:The use of microplate formats facilitates parallel processing of multiple samples, significantly enhancing throughput. Automated liquid handling robots can manage the filling, mixing, and dispensing of microplate wells, allowing for consistent assay conditions and reducing manual labor.
- Buffer Exchange and Purification:Liquid chromatography techniques, such as high-performance liquid chromatography (HPLC) or ultrafiltration, are employed during purification processes to ensure that unwanted components are removed from the vaccine. Accurate liquid handling is critical in loading samples onto columns or membranes to maintain separation efficiency.
- Filling and Packaging:The final stages of vaccine production require precision in filling vials with the formulated product. Automated filling systems must operate under strict aseptic conditions, with mechanisms in place to minimize the risk of contamination during the filling process. Technologies such as weight-based filling or volume-based dispensing ensure that each dose is accurately filled, maintaining the integrity of the product.
Advanced Liquid Handling Technologies
The evolution of liquid handling instruments has been marked by increased automation and integration capabilities. Key advancements include:
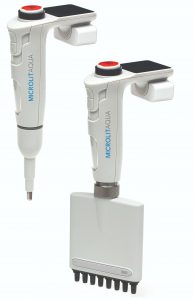 Electronic Pipettes:These electronic pipettes offer programmable settings for volume adjustment and multiple dispensing modes, reducing variability and enhancing accuracy. They can also provide real-time data logging for compliance documentation.
Electronic Pipettes:These electronic pipettes offer programmable settings for volume adjustment and multiple dispensing modes, reducing variability and enhancing accuracy. They can also provide real-time data logging for compliance documentation.- Microfluidics:
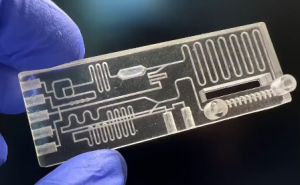 Microfluidic technologies allow for the manipulation of fluids at the microscale, facilitating precise reactions and analyses. This technology is particularly useful in high-throughput screening applications, where small volumes of reagents are used, thereby reducing waste and costs.
Microfluidic technologies allow for the manipulation of fluids at the microscale, facilitating precise reactions and analyses. This technology is particularly useful in high-throughput screening applications, where small volumes of reagents are used, thereby reducing waste and costs. - Robotic Liquid Handling Platforms:These platforms integrate various liquid handling tools and automated systems, enabling seamless workflows from sample preparation to analysis. Their ability to execute complex protocols with minimal human intervention significantly increases productivity and reduces the risk of errors.
Synergistic Integration of Environmental Monitoring and Liquid Handling
Integrating environmental monitoring systems with liquid handling technologies creates a synergistic effect that enhances the overall efficiency and reliability of vaccine development. This integration ensures that liquid handling processes occur within optimal environmental conditions, thereby minimizing contamination risks.
Benefits of Integrated Systems
- Real-Time Environmental Feedback:Automated environmental monitoring systems can provide immediate feedback to liquid handling processes. For example, if temperature or humidity deviates from acceptable ranges, the liquid handling system can automatically pause operations, preventing compromised results.
- Enhanced Compliance and Traceability:Integration facilitates comprehensive data logging, capturing all relevant parameters during the vaccine production process. This not only aids in compliance with regulatory standards but also provides a detailed audit trail for investigations and quality assurance.
- Reduced Contamination Risks:By maintaining optimal environmental conditions during liquid handling operations, the risk of cross-contamination is significantly lowered. This is particularly critical in the aseptic processing of vaccine components, where any introduction of contaminants can lead to severe consequences.
Case Study: Accelerated COVID-19 Vaccine Development
 The COVID-19 pandemic underscored the need for rapid and efficient vaccine development. Leading manufacturers employed state-of-the-art environmental monitoring systems and liquid handling technologies to expedite production while ensuring compliance with stringent safety standards. Continuous monitoring of cleanroom conditions allowed for immediate corrective actions, while automated liquid handling systems enabled rapid scaling of vaccine formulation processes.
The COVID-19 pandemic underscored the need for rapid and efficient vaccine development. Leading manufacturers employed state-of-the-art environmental monitoring systems and liquid handling technologies to expedite production while ensuring compliance with stringent safety standards. Continuous monitoring of cleanroom conditions allowed for immediate corrective actions, while automated liquid handling systems enabled rapid scaling of vaccine formulation processes.
The utilization of these advanced technologies facilitated the production of millions of vaccine doses, highlighting the importance of integrating environmental monitoring with liquid handling in meeting public health demands.
Conclusion
In conclusion, the convergence of effective environmental monitoring and precise liquid handling technologies is crucial in enhancing the efficiency and reliability of vaccine development. As the industry continues to evolve, the adoption of automated, integrated systems will play an increasingly vital role in ensuring the safety, efficacy, and scalability of vaccines. By leveraging these advanced methodologies, pharmaceutical companies can respond to emerging health threats more effectively, paving the way for a safer and healthier future.
Microlit is a leader in liquid handling technology, providing an extensive range of instruments specifically engineered for precision and accuracy in laboratory environments. Emphasizing innovation and quality, Microlit offers advanced solutions, including micropipettes, bottle-top dispensers, electronic burettes, and pipette fillers, each meticulously crafted to deliver optimal performance. To learn more about Microlit products, please email us at info-usa@microlit.com or visit the Microlit products page https://www.microlit.us/shop/.





 4673
4673