ELISA (enzyme-linked immunosorbent assay) is a plate-based assay used to detect and quantify molecular entities, such as antibodies, proteins, peptides and hormones. ELISA is typically performed in 96-well (or 384-well) polystyrene plates, which are able to passively bind antibodies and proteins. Till date, researchers have developed many variants of ELISA for their specific needs, however, all ELISA techniques consist of some basic elements:
- Coating / Capture: This includes direct or indirect immobilization of antigens on the surface of microplate wells.
- Plate Blocking: This step involves the addition of a dissimilar protein onto the microplate well’s surface to cover all unsaturated surface-binding sites. This process happens via hydrophobic interactions between the plastic and non-polar protein residues. Different proteins usually require very specific conditions or pre-treatment for optimal binding. The most popular coating technique involves the addition of a 2-10 μg / ml protein solution dissolved in an alkaline buffer to the microwell plate.
- Probing / Detection: The microplate is incubated with antigen-specific antibodies that bind specifically to the antigens of interest. The plate is generally left to incubate for several hours overnight at 4-37°C.
- Signal Measurement: The signal generated through the direct/indirect tag on the specific antibody is detected and measured. A highly specific antibody-antigen interaction is crucial for an appropriate signal generation.
The ability to wash away non-specific materials makes ELISA a very powerful tool for measuring specific analytes. There are primarily four types of ELISA techniques, namely direct ELISA, indirect ELISA, sandwich ELISA and competitive ELISA, which are discussed in detail below.
-
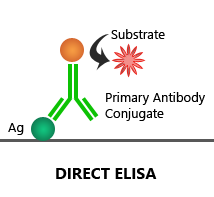
Direct ELISA:
In this assay, the target antigen is coated onto the plate, which is then detected by an enzyme-linked 1′ antibody.
Advantages:
- Easy and quick to perform as no complex steps are involved.
Disadvantages:
- Specificity of the primary antibody may get impacted by the enzyme-linking process
- Scope of signal amplification is limited
- Linking 1′ antibody for every ELISA experiment can be expensive and time-consuming.
-
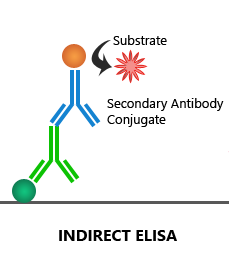
Indirect ELISA:
The target antigen coated on the microplate is first bound by an unconjugated 1′ antibody, which is then detected by an enzyme-linked 2′ antibody.
Advantages:
- Specificity of the 1′ antibody is retained
- Enzyme-linked 2′ antibodies are readily available in the market
- As multiple polyclonal 2′ antibodies can bind to 1′ antibody individually, signal amplification is enhanced.
Disadvantages:
- Risk of cross-reactivity with 2′ antibodies that may result in a non-specific signal.
-
Sandwich ELISA:
Sandwich ELISA requires an antibody pair that targets two distinct epitopes on the target antigen. The first antibody, also called the capture antibody, is coated to the multi-well plate. Then, the target antigen is added, which is detected by the enzyme-linked detection antibody. The target antigen is ‘sandwiched’ between the capture and detection antibodies. Both direct and indirect detection techniques can be used in the sandwich ELISA assay.
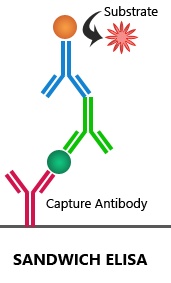
Advantages:
- As the signal detection requires the binding of two 1′ antibodies, the assay has higher specificity.
Disadvantages:
- Commercial pre-prepared kits may not be available readily.
-
Competitive ELISA:
In a competitive ELISA assay, the 1′ antibody is first added to the sample to form antigen-antibody complexes. Then, a sample is added to the microplate well, which is coated with the target antigen. Only the unbound 1′ antibody present in the sample can bind to antigen-coated onto the plate. Based on the amount of antigen present in the sample, more or less free antibodies will be available to bind to the reference antigen, which will, in turn, impact the intensity of the signal. Both direct and indirect detection methods are used in the competitive ELISA technique.
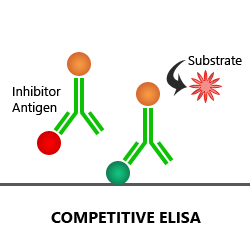
Advantages:
- Higher sensitivity
- Crude sample can be used
Disadvantages:
- Requires 1′ antibodies having high specificity to the sample antigen
Although the overall process of ELISA is quite straightforward, you should take control of certain crucial variables while performing it. For instance, it is important to optimize the plate-coating conditions for the antigen or capture antibody. You should choose an assay microplate, (and not a tissue culture treated plate), which has a minimum protein-binding capacity of 400 ng/cm2. Further, make sure that the coefficient of variation (CV) value of the protein binding is low (<5% is generally preferred). This ensures that the deviation in study results is low.
In addition, it is crucial to remember that you should choose the plate color depending upon the signal being detected. For example, clear polystyrene flat bottom plates are mostly suitable for colorimetric signals, and black or white opaque plates are apt for fluorescent and chemiluminescent signals. In addition, you should also visually inspect plates before you use them, as any scratches or imperfections on the plastic surface are likely to cause aberrations during the detection of the signal. Always make sure that you use high-quality and reliable microwell plates that can generate reproducible results.
To read more informative blogs and articles, visit the page Microlit Blogs.





 9374
9374